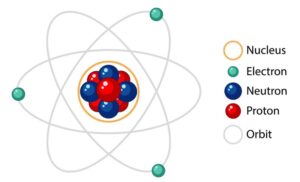
Neutron is one of the fundamental particles constituting the nuclei of the atoms. It is a neutral particle having a mass 1.00898 atomic mass units – nearly equal to that of the proton. It is a spin 1/2 particle and obeys Femi-Dirac statistics.
Discovery of Neutron:
In 1930 Bothe and Becker bombarded beryllium with α-particles and found that a very penetrating radiation was produced. As this radiation was found to be uncharged it was thought to be high energy γ radiation produced according to the nuclear reaction,
4Be9 + 2He4 → 6C13 + hv
The energy of the y-ray was found to be 7 MeV which is greater than the energy of any gamma ray known at that time. In 1932 Curie and Joliot found that when the penetrating radiation from this reaction was made to fall on paraffin wax or other materials containing hydrogen, protons with energies of about 6 MeV were ejected.
It was calculated that to produce protons of 6 MeV the original photon must have· an energy of 60 MeV according to Compton collision of the high energy photon with the proton. An energy of 6 MeV could not be released in the reaction given according to the decrease in mass that took place. Chadwick showed that the experimental results could be explained if we assume that an uncharged and, therefore, highly penetrating particle, having a mass almost equal to that of a proton was emitted in the above reaction as
4Be9 + 2He4 → 6C12 + 0n1
This new particle was given the name neutron (0n1). By the principle of conservation of mass energy, the neutron would be emitted with an energy of 6 MeV and when it strikes against a proton in a head-on collision, the neutron would come to rest and the proton would be released with the energy of 6 MeV.
Chadwick confirmed his hypothesis by the following experiment.
Alpha particles obtained from the source- Polonium (Po) deposited on a disc D were made to bombard a beryllium (Be) target T. Both the disc and the’ target were enclosed in an evacuated chamber B. The neutrons released from the reaction given in (i) escape through the thin wall of the chamber and enter an ionisation chamber C though a thin window W. The ionization chamber is connected to an amplifier and a counter. As the neutrons are uncharged, neutrons themselves cannot produce ionisation in the chamber directly.
Therefore, when the radiations from B entered C, very few pulses per minute were registered. These pulses are due to the ions ejected by the neutrons from the walls of the chamber. On placing a lead sheet about 2 cm thick in the path of the radiation in front of W, it was found that the number of pulses was not reduced appreciably. This showed that the radiations coming out of the Be target were highly energetic.
Further, on introducing a thin sheet of paraffin in place of lead the number of counts increased considerably. This is due to the fact that neutrons eject fast moving protons from paraffin which enter the ionisation chamber through the window W and are counted.
Production of neutron:
The important methods of producing neutron are:
Radium-a-Beryllium source:
This method has been extensively used to provide weak sources of neutrons. In this (α – n) reaction, neutrons are produced by the bombardment of beryllium with high energy alpha particles according to the reaction
4Be9 + 2He4 → 6C12 + 0n1
Photo-disintegration source:
Neutrons can be knocked out from nuclei by high energy photons such as gamma-rays obtained from naturally, or artificially radioactive materials. As for example,
4Be9 + γ → 4Be8 + 0n1
Particle accelerators:
Charged particles accelerated by particle accelerators produce neutrons of definite energy, by bombarding suitable targets. As an example, bombardment of Tritium 1H3 with high energy deutrons or lithium with high energy protons produced neutrons according to the reactions
1H3 + 1D2 → 2He4 + 0n1
3Li7 + 1H1 → 4Be7 + 0n1
From atomic reactors:
The best source of neutrons is an atomic reactor working on the principle of nuclear fission.
Stripping action:
Neutrons may also be obtained when a high energy deutron strikes a target and is stripped of its proton, leaving the neutron alone.
Detection:
Neutrons produce charged particles .by their interaction with matter. The ionization produced by these charged particles is used for detection of neutrons. The kinetic energy of the neutrons can also be measured by these methods after suitable calibration of the experiment.
Ionisation chamber method:
The ionisation chamber is filled with a gaseous boron compound such as BF3 (Boron trifluoride) or lined with a solid boron compound. When a neutron strikes boron, it liberates alpha-particles according to the reaction.
5B10 + 0n1 → 3Li7 + 2He4
The alpha-particle is detected as usual by the ionisation chamber.
Induced radioactivity method:
Many materials become radioactive under neutron bombardment and, can serve as neutron detectors, e.g., when a neutron strikes a silver foil, the new silver isotope formed is radio-active. The nuclear reaction that takes place is
47Ag107 + 0n1 → 47Ag108 + γ
The product decays according to the reaction
47Ag108 → 48Cd108 + -1e0
The beta-particle emitted can be detected and its energy measured.
Elastic collision method:
In this method the ionisation chamber is filled with hydrogen or is provided with a window made of hydrogenous material. The neutron makes an elastic collision with a proton and is brought to rest. The proton moves forward with the same energy and is detected by the ionisation it produces or by any suitable method.
Fast, slow and thermal neutrons:
Neutrons with energies above 1.2 MeV are called fast neutrons, because 1.2 MeV is the minimum energy for fission of 92U235. These neutrons have energies upto 10 MeV. Many nuclear reactions which are energetically not possible at lower neutron energies take place with fast neutrons.
Neutrons within the energy interval 10-50 MeV are called very fast neutrons and those beyond 50 MeV are called ultra-fast or ultra-high energy neutrons. Neutrons with energies below 1 eV are called slow neutrons.
Neutrons with energy between 1 eV and 1.2 MeV are called epithermal neutrons. Those neutrons which are in thermal equilibrium with the matter through which they pass and have a Maxwellian distribution of energies with a most probable value depending upon temperature are called thermal neutrons. The most probable energy of thermal neutron at 27°C is 0.025eV. When neutrons pass through a material, they are slowed down by collisions with the nuclei of the material and lose a· part of their energy.
For heavier nuclei the loss is very small and a very large number of collisions are required to slow down the· neutron. For light nuclei the loss is large and neutrons are slowed down quickly. Materials rich in hydrogen are very efficient in slowing neutrons and are known as moderators. Cadmium has the property of absorbing neutrons and is known as absorber.
Almost all elements can be transmuted by neutrons. Some of the elements require fast neutrons whereas others require slow ones. Thus, a fast neutron ejects an alpha-particle from a nitrogen nucleus thereby converting it into a boron nucleus.